Answer: The correct answer is two cobalt atoms.
Step-by-step explanation:
Cobalt (III) oxide is a compound which is formed by the combination of cobalt and oxygen atoms.
The oxidation state of cobalt in the compound is +3 and the oxidation state of oxygen in the compound is -2.
Thus, by criss-cross method, the oxidation states of the ions becomes the subscipt of the other atom which represents the number of individual atoms in a compound. So, the chemical formula of cobalt (III) oxide becomes
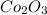
In the given compound, 2 atoms of cobalt are present and 3 atoms of oxygen are there.
Thus, the correct answer is two cobalt atoms.