Answer:
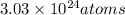 of carbon in the sample.
of carbon in the sample.
73.7960 grams is the total mass of the sample.
Step-by-step explanation:
Compound =
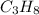
Total numbers of hydrogen atoms in a compound =
 atoms
atoms
In one molecule of compound there are 8 hydrogen atoms.
Then x number of molecules of compound having
 hydrogen atoms.
hydrogen atoms.
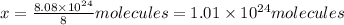
In one molecule of compound there are 3 carbon atoms.
Then
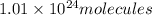 of given compound will have :
of given compound will have :
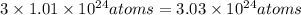 of carbon
of carbon
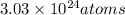 of carbon in the sample.
of carbon in the sample.
Moles of given compounds = n
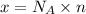
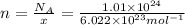
n = 1.6771 moles
Mass of 1.6771 moles of given compound:
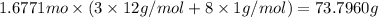
73.7960 grams is the total mass of the sample.