If light of sufficient frequency falls on the metal surface then due to this energy of light electrons will move out of the metal surface
this phenomenon is known as photoelectric effect
As per Einstein's equation we know that
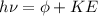
here we know that
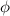 = work function
= work function
so if light have sufficient frequency so that the work function of metal is less than the energy of photon then it will surely eject the electrons and photo electric effect will occur
So here correct answer will be
A) Electrons are emitted as soon as low-intensity, high-frequency light hits a metal surface.