Answer:
The pH of the solution is 1.
Step-by-step explanation:
Concentration of HCl solution = 0.10 M
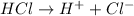
1 mole of HCl gives 1 mole of
 ions.
ions.
![[HCl]=[H^+]=0.10 M](https://img.qammunity.org/2018/formulas/chemistry/high-school/2cljlzbx81t1wydvddfymntwpxtvcragz7.png)
The pH of the solution is defined as negative logarithm of
 ions in the solution.Mathematically written as
ions in the solution.Mathematically written as
![pH=-\log[H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/9so9u3mmeurzbcqkpf3jt5b89ctmc03tdp.png)
![pH=-\log[0.10 M]=1](https://img.qammunity.org/2018/formulas/chemistry/high-school/oakp42putdauvojckxf3644w2kc7gzxenl.png)
The pH of the solution is 1.