Answer:
37.5 g
Step-by-step explanation:
First you should kwow the molar mass of the lithium, so you can use your periodic table.
Molar mass of lithium =
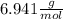
Then you should relate the molar mass of the lithium with the quantity of moles the problem gives you:
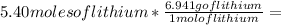 37.48 g of lithium
37.48 g of lithium
Rounding the answer
37.5 g of lithium