First a balanced reaction equation must be established:
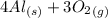
→
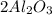
Now if mass of aluminum = 145 g
the moles of aluminum = (MASS) ÷ (MOLAR MASS) = 145 g ÷ 30 g/mol
= 4.83 mols
Now the mole ratio of Al : O₂ based on the equation is 4 : 3
[
4Al +
3 O₂ → 2 Al₂O₃]
∴ if moles of Al = 4.83 moles
then moles of O₂ = (4.83 mol ÷ 4) × 3
=
3.63 mol (to 2 sig. fig.)
Thus it can be concluded that
3.63 moles of oxygen is needed to react completely with 145 g of aluminum.