Answer:
The correct answer is:
An electron will be emitted in the second experiment, but it cannot be determined whether it will reach the second plate.
Step-by-step explanation:
In fact, violet has higher frequency than green light. This means that photons on violet carry more energy than photons of green light (remember that the energy of a photon is proportional to it's frequency:
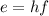
, so when they hit the surface of the metal, more energy is transferred to the electrons. The electron was already emitted with green light, so it must be emitted with also violet light, given the more energy transferred.