Answer:
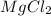
Explanation:

According to stoichiometry,
1 mole of
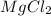 reacts with 2 moles of
reacts with 2 moles of
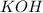
Thus when 1 mole of
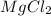 is added to 3 moles of
is added to 3 moles of
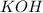 , (3-2)moles= 1 mole of
, (3-2)moles= 1 mole of
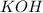 will remain unused.
will remain unused.
Thus
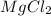 is the limiting reagent as it decides the formation of products.
is the limiting reagent as it decides the formation of products.
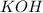 is excess reagent as it remains unused.
is excess reagent as it remains unused.