Answer : The correct option is, 0.188 M
Solution : Given,
Volume of HBr solution = 27.3 ml = 0.0273 L (1 L = 1000 ml)
Molarity of HBr solution = 0.293 M
Volume of
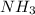 solution = 42.6 ml = 0.0426 L
solution = 42.6 ml = 0.0426 L
The complete neutralization reaction is,
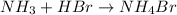
Formula used :
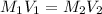
where,
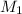 = Molarity of HBr solution
= Molarity of HBr solution
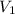 = volume of HBr solution
= volume of HBr solution
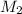 = Molarity of
= Molarity of
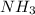 solution
solution
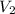 = volume of
= volume of
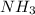 solution
solution
Now put all the given values in the above formula, we get the concentration of
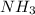 .
.
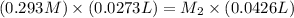
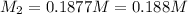
Therefore, the concentration of the
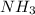 is, 0.188 M
is, 0.188 M