Hello!
Which equation is set up correctly to determine the volume of a 1.5 mole sample of oxygen gas at 22°C and 100 kPa?
We have the following data:
v (volume) = ? (in L)
n (number of mols) = 1,5 mol
T (temperature) = 22 ºC
First let's convert the temperature on the Kelvin scale, let's see:
TK = TºC + 273,15
TK = 22 + 273,15
TK = 295,15
P (pressure) = 100 kPa → P = 100000 Pa → P ≈ 0,987 atm
R (gas constant) = 0,082 atm.L / mol.K
We apply the data above to the Clapeyron equation (gas equation), let's see:
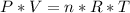
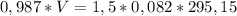
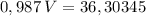
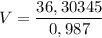

_______________________
I Hope this helps, greetings ... Dexteright02! =)