Half-life is defined as the amount of time it takes a given quantity to decrease to half of its initial value. The equation to describe the decay is
Nt=N0(1/2)
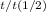
where N0 is the initial quantity, Nt is the remaining quantity after time t, t1/2 is the half-time. So work out the equation, t1/2 = t (-ln2)/ln(Nt/N0) = 11.5*(-ln2)/ln(12.5/100) = 3.83 days