Given :
Number of atoms of an element, n = 2.072 × 10⁴³ atoms.
To Find :
Number of moles of that element in given number of atoms.
Solution :
We know, 1 mole of any element contains 6.022 × 10²³ atoms.
So, number of moles in given number of atoms are :
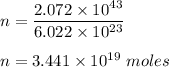
Hence, this is the required solution.