Answer:
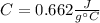
Step-by-step explanation:
Hello!
In this case, since the heat removal when cooling down a body is computed via:
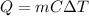
Given the removed heat, mass and temperature change, we solve for the heat capacity of the metal as shown below:
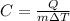
Thus, by plugging in we obtain:
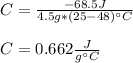
Best regards!