Answer:
b . 0.351 L.
Step-by-step explanation:
Hello!
In this case, since diluted solutions are prepared by adding an extra amount of diluent to a stock-concentrated solution, we infer that the number of moles of solute remains the same, therefore we can write:
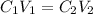
Thus, solving for the volume of the stock solution, V1, we obtain:
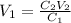
Now, by plugging in the given data we obtain:
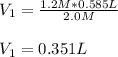
Therefore, the answer is b . 0.351 L.
Best regards!