Answer:
3d
Step-by-step explanation:
Transition metal are elements having partially filled d sub shell. In these metals last electron is filled in d sub shell.
General electronic configuration of transition metal:
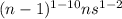
Where, n = outermost shell.
Period 4 means, last shell no. is 4.
For transition metals of period 4,
General electronic configuration:
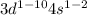
for example, Fe belong to periods 4.
Electronic configuration of Fe:
![[Ar]3d^64s^2](https://img.qammunity.org/2018/formulas/chemistry/high-school/khan8acn32jp263cz1rq0cka3eqsoyqpu6.png)
So, filling of 3d orbital is represented by transition metal of 4.