Answer : The correct option is, 40 %
Explanation: Given,
Molar mass of C = 12.01 g/mole
Molar mass of H = 1.0079 g/mole
Molar mass of O = 16.00 g/mole
First we have to calculate the molar mass of glucose.
Molar mass of glucose
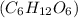 =
=
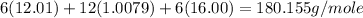
Now we have to calculate the percent composition of carbon in glucose.
As we now that there are 6 number of carbon atoms, 12 number of hydrogen atoms and 6 number of oxygen atoms.
The mass of carbon =
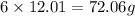
Formula used :
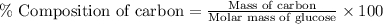
Now put all the given values in this formula, we get the percent composition of carbon in glucose.
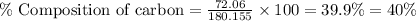
Therefore, the percent composition of carbon in glucose is, 40 %