Answer: The oxygen is coming from the atmosphere.
Step-by-step explanation: After Nitrogen gas, the most abundant gas present in the atmosphere is oxygen gas which makes it available for the reaction.
The product which forms after the reaction between copper metal and oxygen gas is copper oxide which is a black color coating on the surface of the copper.
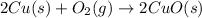
When this copper oxide reacts with hydrogen gas, then the reaction among these two reactant gives copper metal and water as products.
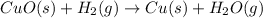
From the above reaction, the formation of water shows that the oxyegn atom came from CuO.