Answer:
There are 316.46 g of Cl in 495 g of CaCl₂.
Step-by-step explanation:
- The molar mass of CaCl₂ is 40 + 35.45 + 35.45 = 110.9 g/mol
- Moles of CaCl₂ present in 495 g of CaCl₂ = 495 g ÷ 110.9 g/mol = 4.46 mol CaCl₂
With that data we can calculate the moles of Cl, and then convert them into grams:
4.46 mol CaCl₂ *
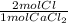 *
*
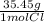 = 316.46 g of Cl
= 316.46 g of Cl