Answer:
c. One sodium atom with 1 valence electron and 1 chlorine atom with 7 valence electrons
Step-by-step explanation:
The electronic configuration of one sodium atom is
Na = 2, 8, 1
The electronic configuration of one chlorine atom is
Cl = 2, 8, 7
Therefore Na atom has 1 electron in its valence shell whereas Cl atom has 7 electron in its valence shell.
So Na atom combines with Cl atom to form NaCl atom by transferring atom from the Na atom to Cl atom forming
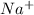 ion and
ion and
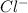 ion
ion
Thus Na and Cl react to form ionic bond.