Answer: Option (a) is the correct answer.
Step-by-step explanation:
The reaction will be as follows.
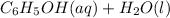

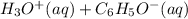
Here, phenol is acting as an acid as it's conjugate base is more stabilized due to resonance. Whereas if
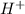 ion is donated by water molecule then it will give a hydroxide ion which is not a strong base.
ion is donated by water molecule then it will give a hydroxide ion which is not a strong base.
Therefore, here phenol is acting as an acid as it is donating an
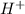 ion.
ion.