Answer: Option (d) is the correct answer.
Step-by-step explanation:
Since the given formula is
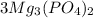 . According to cross method formula, magnesium has +2 charge so,
. According to cross method formula, magnesium has +2 charge so,
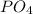 is multiplied by 2.
is multiplied by 2.
Thus, 1 molecule of magnesium phosphate will contain 2 atoms of phosphorus.
Therefore, three molecules of magnesium phosphate contains following number of atoms.
Hence, we can conclude that there are 6 atoms of phosphorus in three molecules of magnesium phosphate,
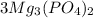 .
.