Answer: oxygen
Step-by-step explanation:
An ionic compound is formed when one element completely transfers its valence electron to another element.
The element which donates the electron is known as electropositive element and forms a positively charged ion called as cation. The element which accepts the electrons is known as electronegative element and forms a negatively charged ion called as anion.
As alkaline earth metal can lose two electrons, it will combine with an element which can gain electrons.
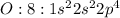
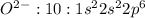
Thus alkaline earth metal MOSTLY LIKELY combine with oxygen.