Answer:
Heat is required to increase the temperature of 35.0 grams of water is 5,120.5 J.
Step-by-step explanation:
Mass of water = m = 35.0 g
Change in temperature of the water[=ΔT = 45.0°C to 10.0°C = 35.0°C
The specific heat of water = c = 4.18 J/g°C
Heat is required to increase the temperature of 35.0 grams of water be Q.
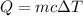
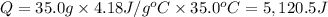
Heat is required to increase the temperature of 35.0 grams of water is 5,120.5 J.