Answer : The number of moles of hydrogen gas is, 0.033 mole
Explanation : Given,
Moles of sodium = 0.066 mole
The balanced chemical reaction will be,
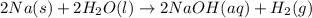
From the balanced chemical reaction, we conclude that 2 moles of sodium react with 2 moles of water to give 2 moles of sodium hydroxide and 1 mole of hydrogen has as a product.
As, 2 moles of sodium react to give 1 mole of hydrogen gas
So, 0.066 mole of sodium react to give
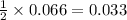 moles of hydrogen gas
moles of hydrogen gas
Therefore, the number of moles of hydrogen gas is, 0.033 mole