Answer:
If the two fatty acids contain an equal number of carbon atoms, the saturated fatty acid would contain more hydrogen atoms than the unsaturated one.
Step-by-step explanation:
A fatty acid molecule is made up of two parts:
- an aliphatic hydrocarbon chain (possibly very long,) and
- a carboxyl group (
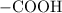 .)
.)
The fatty acid is considered "saturated" if the hydrocarbon chain part contains only single carbon-carbon bonds. If the hydrocarbon chain of that fatty acid contains carbon-carbon double or triple bonds (such as
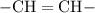 and
and
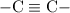 ,) that fatty acid would be "unsaturated."
,) that fatty acid would be "unsaturated."
Each carbon atom typically needs four covalent bonds. For every additional carbon-carbon bond in the chain, one carbon-hydrogen bond would need to be deleted from both sides. Thus, adding one carbon-carbon bond would reduce the number of hydrogen atoms in the acid by two. For example:
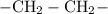 (single bonds only) includes four hydrogen atoms.
(single bonds only) includes four hydrogen atoms. - An extra carbon-carbon bond would bring the number of hydrogen atoms down by two:
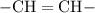 .
. - Another carbon-carbon bond would again bring that number by two more:
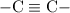 .
.
Assume that the unsaturated fatty acid includes the same number of carbon atoms as the saturated fatty acid. The unsaturated fatty would include more carbon-carbon bonds, fewer carbon-hydrogen bonds, and therefore fewer hydrogen atoms overall.