Answer:
a.0.25 g
b.0.16 g
Explanation:
We are given that
Half-life of the element radon=3.8 days
Mass of sample of radon=1 g
The amount of radon left after t days is given by
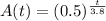
a.
We have to find the amount of radon left after 7.6 days.
Substitute t=7.6 in the equation
Then, we get
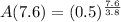
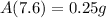
Hence, the amount of radon will be present after 7.6 days=0.25 g
b. We have to find the amount of radon left after 10 days.
Substitute t=10 in the equation
Then, we get
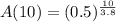
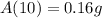
Hence, the amount of radon will be present after 10 days=0.16 g