Answer:The reaction is exothermic.
Step-by-step explanation:
Enthalpy of Formation (
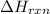 ): It is defined as change in enthalpy when one mole of substance is formed at standard temperature and pressure from its pure elements under same conditions.
): It is defined as change in enthalpy when one mole of substance is formed at standard temperature and pressure from its pure elements under same conditions.
1)
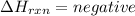
When the energy is released during formation of compound in a chemical reaction that is an Exothermic reaction.
2)
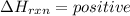
When the energy is absorbed during the formation of compound in a chemical reaction that is an Endothermic reaction
The
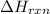 of formation of carbon dioxide is negative means that the reaction is exothermic reaction.
of formation of carbon dioxide is negative means that the reaction is exothermic reaction.