Answer: Option (a) is the correct answer.
Step-by-step explanation:
Enthalpy is defined as the total amount of heat present in a system.
When there is bond formation in a chemical reaction then it means energy is released. Hence, the reaction is exothermic in nature and
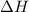 < 0 or negative.
< 0 or negative.
On the other hand, when breaking of bonds occurs then energy is absorbed by the system. Hence, the reaction is endothermic in nature and
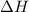 > 0 or positive.
> 0 or positive.
Thus, in the given reaction
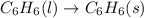 , the state is changing from liquid to solid.
, the state is changing from liquid to solid.
This means atoms are coming close to each other and hence, there is occurrence of bond formation. As a result, energy will be released to the surroundings.
Hence, we can conclude that H < 0 for the given reaction.