Answer: B )
![H_2O]](https://img.qammunity.org/2018/formulas/chemistry/high-school/mb9img87g4fg9rwupyyzdjzoea3uhrisgn.png)
Explanation:- According to the Bronsted Lowry conjugate acid-base theory, an acid is defined as a substance which donates protons and a base is defined as a substance which accepts protons.
Amphoteric substances are substances which can act as both acids and bases and thus can accept and donate
 ions.
ions.
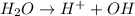
Here water acts as an acid as it donates
 ions.
ions.
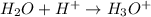
Here water acts as a base as it accepts
 ions.
ions.