Answer: the same
Explanation:
According to the dilution law,
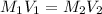
where,
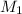 = molarity of stock solution = 0.50 M
= molarity of stock solution = 0.50 M
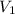 = volume of stock solution = 10 ml= 0.01 L (1L=1000ml)
= volume of stock solution = 10 ml= 0.01 L (1L=1000ml)
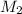 = molarity of resulting solution = ? M
= molarity of resulting solution = ? M
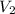 = volume of resulting solution = 100 ml = 0.1 L
= volume of resulting solution = 100 ml = 0.1 L
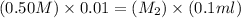
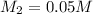
Therefore, the Molarity of resulting solution is 0.05 M
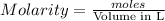
1. when volume is 10 ml or 0.01L (1L=1000ml)
moles of
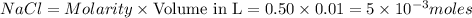
2. when volume is 100 ml or 0.1L (1L=1000ml)
moles of
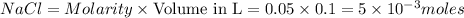
Thus they contain same amount of
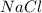 .
.