Answer: 2)

Explanation: When an acid reacts with the base then it undergoes neutralization to form a salt and water.
1)
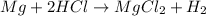 : is an example of single replacement reaction as Mg replaces hydrogen from its salt solution.
: is an example of single replacement reaction as Mg replaces hydrogen from its salt solution.
2)
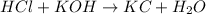 : is an example of neutralization where acid and base reacts to give salt and water.
: is an example of neutralization where acid and base reacts to give salt and water.
3)
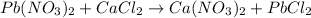 : is an example of double displacement reaction in which exchange of ions take place.
: is an example of double displacement reaction in which exchange of ions take place.
4)
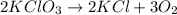 : is an example of decomposition where a single reactant decomposes to give two or more products.
: is an example of decomposition where a single reactant decomposes to give two or more products.