The final temperature : T₂=209.86 K = -63.1 °C
Further explanation
Given
V₁=568 cm³
T₁=25° C+ 273 = 298 K
V₂=400 cm³
Required
Final temperature(T2)
Solution
Charles's Law
When the gas pressure is kept constant, the gas volume is proportional to the temperature
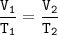
T₂=(V₂.T₁)/V₁
T₂=(400.298)/568
T₂=209.86 K = -63.1 °C