The atoms combine with other atoms in order to complete their octet (eight electrons in their valence shell, noble gas configurations) and attain stability. The combination of atoms with other atoms to form molecule takes place by either sharing of electrons or by transfer of electrons from one atom to other atom.
Fluorine,
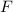 and bromine,
and bromine,
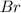 are in the same group on the periodic table that is halogen group (Group 17). The halogen group has electronic configuration as
are in the same group on the periodic table that is halogen group (Group 17). The halogen group has electronic configuration as
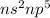 that means they are deficient of one electron to complete their octet and attain stability. So, they gain 1 electron and result in the formation of anion and form compounds by transfer of electrons.
that means they are deficient of one electron to complete their octet and attain stability. So, they gain 1 electron and result in the formation of anion and form compounds by transfer of electrons.
Hence, both a fluorine atom and a bromine atom gain one electron, and both atoms become stable.