The question is incomplete. Here, is the complete question:
A balloon has a volume of 253.2 mL at 356 K. The volume of the balloon is decreased to 165.4 mL. What is the new temperature?
Answer: The new temperature is 232.6 K.
Step-by-step explanation:
Charles' Law: This law states that volume is directly proportional to the temperature of the gas at constant pressure and number of moles.
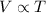 (At constant pressure and number of moles)
(At constant pressure and number of moles)
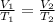
where,
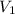 = initial volume of gas = 253.2 ml
= initial volume of gas = 253.2 ml
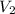 = final volume of gas = 165.4 ml
= final volume of gas = 165.4 ml
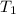 = initial temperature of gas= 356 K
= initial temperature of gas= 356 K
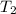 = final temperature of gas = ?
= final temperature of gas = ?
Putting in the values:
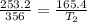
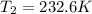
Therefore, the new temperature is 232.6 K.