Answer:
0489 M is the concentration of HCl in the original 10.00 mL sample.
Step-by-step explanation:

To calculate the concentration of acid, we use the equation given by neutralization reaction:
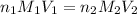
where,
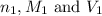 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is
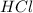
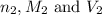 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.
We are given:
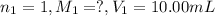
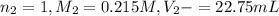
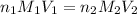
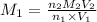
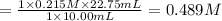
0489 M is the concentration of HCl in the original 10.00 mL sample.