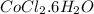Answer : The formula for the
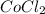 hydrate is
hydrate is
Explanation : Given,
Mass of hydrate of
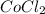 = 6.00 g
= 6.00 g
Mass of
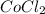 = 3.27 g
= 3.27 g
First we have to calculate the mass of water.
Mass of water = Mass of hydrate of
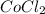 - Mass of
- Mass of
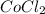
Mass of water = 6.00 - 3.27 = 2.73 g
Now we have to calculate the moles of
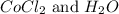
Molar mass of
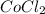 = 129.8 g/mole
= 129.8 g/mole
Molar mass of
 = 18 g/mole
= 18 g/mole
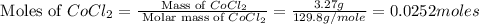
and,
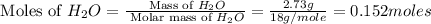
Now we have to calculate the mole ratio of the given molecules.
For the mole ratio, we divide each value of the moles by the smallest number of moles calculated which is 0.0252 moles.
For
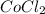 =
=
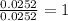
For
 =
=
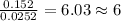
The ratio of
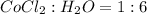
Hence, the formula for the
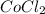 hydrate is
hydrate is