Answer : The concentration of solution in percent by mass is 16.3 %
Explanation :
Percent by mass : It means that the mass of solute present in the mixture and multiply by 100.
Given:
Mass of LiCl = 35.8 g
Mass of water = 184 g
First we have to calculate the mass of mixture.
Mass of mixture = Mass of LiCl + Mass of water
Mass of mixture = 35.8 g + 184 g
Mass of mixture = 219.8 g
Now we have to calculate the concentration of solution in percent by mass.
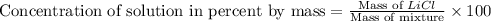
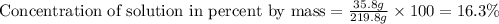
Therefore, the concentration of solution in percent by mass is 16.3 %