Answer:
The energy of a photon is
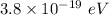 .
.
Step-by-step explanation:
Given that,
Wave length = 520 nm
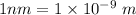
Using plank's equation
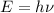 ...(I)
...(I)
Here, h = plank constant
υ = frequency
Now, using wave equation
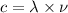
The equation can be written as:
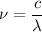
Here, c = speed of light
λ= wave length of light
Put the value of υ in equation (I)
The energy of photon is
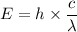
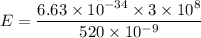
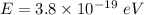
Hence, The energy of a photon is
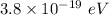 .
.