Answer:
m = 16.110 g
Step-by-step explanation:
As we know that error in the measurement is the deviation in the result obtained from experimental result and theoretical result.
So here we will find the error in each reading as we know that theoretical result of the mass is 16.100 g
so here we will have
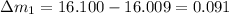
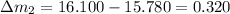
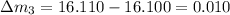
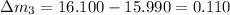
So in all above the least error is in the mass 16.110 g reading