Answer:
the heat emitted by potatoes is much less than the heat emitted by turkey
Step-by-step explanation:
The heat transfer is given by
Q = m
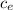 ΔT
ΔT
where m is the mass of the body, c_e is the specific heat and ΔT is the change in temperature of the body
in this case they ask us to appear the heat emitted by the potatoes and the turkey.
From the tables found the specious colors of both
potato c_e = 0.82 Kcal / kg ºC
turkey c_e = 0.72 Kcal / kg ºC
Since the two bodies have the same initial temperature and we assume that they reach the same final (ambient) temperature, the ratio of the heat emitted is
Q₁ / Q₂ =
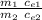
where we use the rise 1 for the potatoes and the su index 2 for the turkey
the mass of the potatoes can be estimated at approximately m = 1 ka
we substitute
Q₁ / Q₂ =
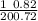
Q₁ /Q₂ = 0.0569
Q₁ = 0.0569 Q₂
therefore the heat emitted by potatoes is much less than the heat emitted by turkey