Answer: The group number of X is Group 1.
Step-by-step explanation:
Groups are defined as the vertical columns in a periodic table.
We are given a chemical compound having chemical formula of
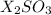
This is formed by the combination of X atoms and
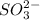 ions.
ions.
By criss-cross method, 2 atoms of X combine with 1 atom of sulfite ions.
Thus, the oxidation state of X atom is +1.
The elements belonging to Group 1 shows an oxidation state of +1.
Hence, the group number of X is Group 1.