Answer: The correct answer is fusion reaction.
Step-by-step explanation:
Nuclear fission reaction is defined as the reaction in which a heavier nuclei dissociates into two or more lighter nuclei.
Nuclear fusion reaction is defined as the reaction in which lighter nuclei combines to form a heavier nuclei.
Chemical reaction is defined as the reaction in which change in composition takes place. A new substance gets formed in this type of reaction.
The chemical equation for the formation of helium-4 atom follows:
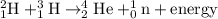
In the above reaction, two hydrogen nuclei are combining together to form a heavier nuclei which is helium atom.
Thus, the correct answer is fusion reaction.