Step-by-step explanation:
In a reduction-half reaction, there is gain of electrons.
For example,
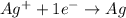
Whereas in an oxidation-half reaction, there is loss of electrons.
For example,
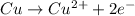
Thus, we can conclude that the equation Cu → cu2 2e– represents oxidation-half reaction.