Answer:
The work done will be
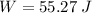
Step-by-step explanation:
The work equation is given by:
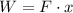
Where:
F is the force due to gravity (weight = mg)
x is the length of the ramp (3 m)
Now, the force acting here is the component of weight in the ramp direction, so it will be:
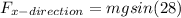
Therefore, the work done will be:
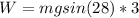
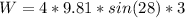
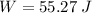
I hope it helps you!