Answer:
The answer is 0.67 M
Step-by-step explanation:
Sucrose solution molarity=
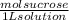
You have to divide the mass of sucrose (171 g) by the molecular weight of sucrose (342 g/mol) and then divide the result by the volume in liters (0.750 L) as follows:
Molarity=
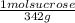 x
x
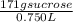
Molarity= 0.666 mol sucrose/L
Molarity= 0.67 M