Step-by-step explanation:
1. Cesium (Cs) is a group 1 element and it has electronic configuration
![[Xe] 6s^(1)](https://img.qammunity.org/2018/formulas/chemistry/high-school/jmyi3asyio5d8i3e2to58on15qvah02wf0.png) .
.
On the other hand, bromine is a group 7 element and it has electronic configuration
![[Ar] 3d^(10)4s^(2)40^(5)](https://img.qammunity.org/2018/formulas/chemistry/high-school/zvm6lt8mvlruddqdj1w4dzxtek2bxbh5mx.png) .
.
Since, Cs is a metal so it will readily lose 1 electron but bromine being a non-metal needs 1 electron to completely fill its orbital.
Hence, bromine atom will attract electrons more strongly.
2. Sodium (Na) is a a group 1 element and it has electronic configuration
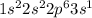 .
.
Whereas, chlorine is a group 7 element and it has electronic configuration
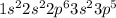 .
.
So, sodium is a metal hence, it readily loses its one electron and chlorine being a non-metal will attract one electron in order to completely fill its orbital.
Thus, we can conclude that sodium (Na) atom loses an electron more readily.