Answer:The new volume when 0.40 moles of gas are added to the balloon is 8 L.
Step-by-step explanation:
Initial moles of gas
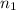 = 0.10 moles
= 0.10 moles
initial volume of the balloon,
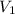 = 2.0 L
= 2.0 L
Final moles of gas
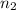 = 0.40 moles
= 0.40 moles
Final volume of the balloon,
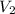 = 2.0 L
= 2.0 L
Avogadro Law:
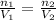
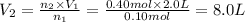
The new volume when 0.40 moles of gas are added to the balloon is 8 L.