The concentration in mol/L of the chemist's potassium iodide solution : = 4.4 x 10⁻⁴ M
Further explanation
Given
88. μmol of Potassium iodide
200. mL volumetric flask
Required
The concentration
Solution
Molarity is a way to express the concentration of the solution
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
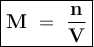
Conversion :
88. μmol = 88 . 10⁺⁶ mol = 8.8 10⁻⁵ mol
200 ml = 0.2 L
The molarity :
= n : V
= 8.8.10⁻⁵ : 0.2
= 4.4 x 10⁻⁴ M