Answer: Option (C) is the correct answer.
Step-by-step explanation:
A single replacement reaction is defined as the reaction where an element upon reaction with a compound tends to displace another element in that compound.
For example,
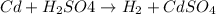 is a single replacement reaction.
is a single replacement reaction.
And, when number of atoms on reactant side equal to the number of atoms on product side then it is known as a balanced chemical reaction.
Thus, we can conclude that
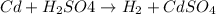 represents a balanced, single replacement chemical reaction.
represents a balanced, single replacement chemical reaction.