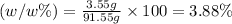Answer:
The mass percentage of the solution is 3.88%.
Step-by-step explanation:
Percentage by mass (w/w%) : The percentage mass or fraction of mass of the of solute present in total mass of the solution.
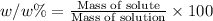
Mass of solute that is NaCl = 3.55 g
Mass of solvent that is water = 88 g
Mass of the solution = Mass of solute + Mass of solvent
= 3.55 g + 88 g = 91.55 g
Solution's percentage by mass: